Tech & Tools
Technical information
- HOME
- Tech & Tools
- Ion exchange resin
Ion exchange resin
Tap water, which is necessary for daily life and factory water, is very transparent and has been filtered cleanly. However, this clear water contains ions such as sodium, calcium and chlorine, and colloidal silica, which has a negative surface charge.
Positively charged ions such as calcium ion, magnesium ion, and sodium ion are called cations, and negatively charged ions such as sulfate ion, chlorine ion, and bicarbonate ion are anions (cations). It is called an anion).
What is contained in groundwater as ions cannot be removed by physical filtration such as filtered sand or anthracite. However, ion exchange resins are filter media that can remove these components by exchanging these ions.
An ion exchange resin is an ion exchanger that exhibits an ion exchange phenomenon. Ion exchange differs depending on the group (functional group) bonded to the parent synthetic resin, and those having acidic groups such as acidic hydroxyl groups and carboxyl groups and capable of exchanging cations are cation exchange resins (or cation exchange resins), and vice versa. Those that have a basic group such as an amino group or an imino group and can exchange anions are called anion exchange resins (or anion exchange resins).
As the name implies, the mechanism of ion exchange in an ion exchange resin is the exchange of ions bound to the functional groups of the ion exchange resin and ions in raw water.
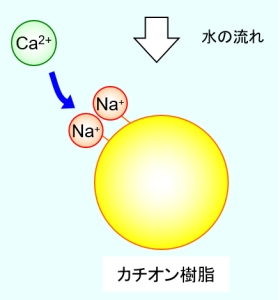
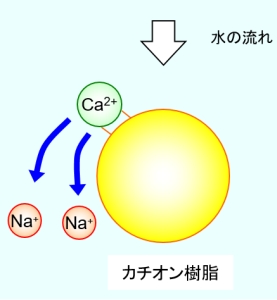
For example, in the case of a cation exchange resin used in a water softener, the hardness of the raw water can be removed by exchanging the sodium ion bonded to the resin with the calcium ion or magnesium ion in the raw water as shown in the figure below. is. Depending on the intended use of the treated water, a cationic resin with hydrogen ions bonded may be used. In the case of anion exchange resin, anions such as chlorine ions and hydroxyl ions bonded to functional groups are exchanged with the anions that you want to remove.
However, if ion exchange is continued as shown in the above figure, sodium ions bound to the resin will be exchanged for other ions, and ion exchange will gradually become impossible. The maximum amount of ion exchange that an ion exchange resin has is called the total capacity, and if it exceeds this, it will not be possible to exchange with the ions that are the target of removal of raw water.
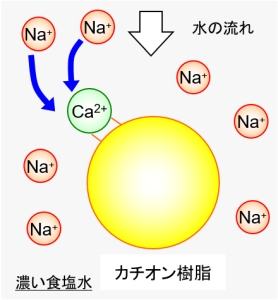
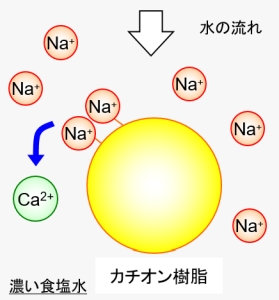
In such a case, it is possible to exchange calcium ions with the initial sodium ions again by contacting them with a large amount of sodium ions. Generally, by rinsing with a cheap and easy-to-use saline solution, the captured calcium ions will be exhaled. This is called “regeneration” of the ion exchange resin.
Head Office
1-12-11 Tagawakita, Yodogawa-ku,Osaka
532-0021
Overseas Business Department
TEL +81-6-6301-6460
FAX +81-6-6308-3022